Lohocla Research Development Pipeline
Nezavist: Brief Summary
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) describes Alcohol Use Disorder (AUD) as a “chronic, relapsing brain disease characterized by an impaired ability to stop or control alcohol use despite adverse social, occupational, or health consequences.” As of 2015, AUD affected 15.1 million adults and 623,000 adolescents in the U.S., and data from 2010 estimate an economic burden of $249 billion in the U.S. Globally, alcohol misuse was the fifth leading risk factor for premature death and disability in 2010.
Neurobiological hypotheses of AUD/alcohol addiction postulate that neuroadaptations occur in brain neurotransmitter systems that contribute to “craving” for alcohol, and relapse to drinking by dependent individuals who have stopped or reduced drinking for a period of time. Lohocla Research designed Nezavist to target these neuroadaptive changes and reduce relapse drinking. Lohocla Research has received clearance from the FDA to begin first-in-human trials.
Lohocla Research’s laboratories have contributed critical information to the understanding of the mechanisms by which alcohol affects behavior and the changes caused in brain by chronic alcohol ingestion that results in alcohol addiction. A discussion of this work can be found in a publication by Tabakoff and Hoffman in the journal Pharmacology, Biochemistry and Behavior.
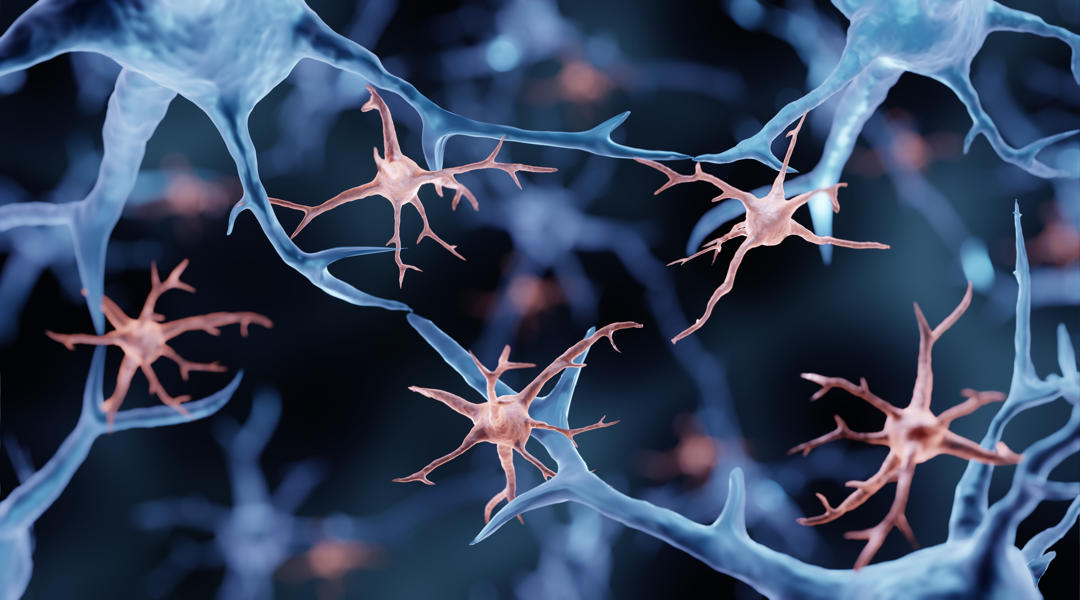

Hyper-ramification is the principal form of microglial activation in response to chronic alcohol consumption. Repeated cycles of drinking result in increasingly sensitized/activated hyper-ramified microglia, which contributes to the neurobiology of substance use disorders (adapted from F.T. Crews et al., 2017).
However, the most current research has demonstrated that a more holistic mechanism is
responsible for human
and other animal alcohol drinking behavior. Recent work shows that alcohol's actions in the gut may be the
initial site that instigates the biological changes that lead to AUD.
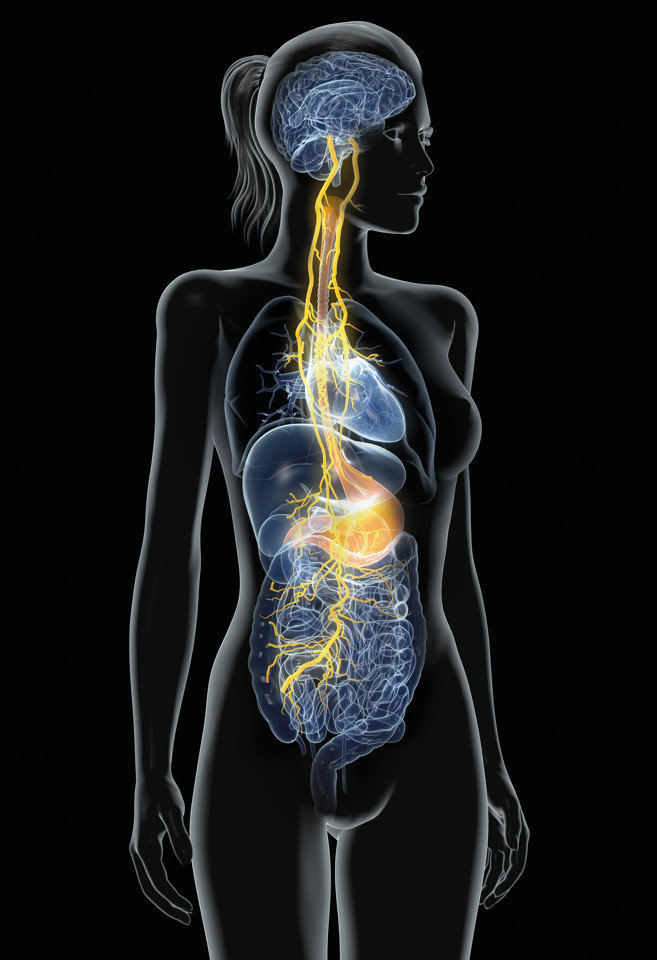
Nezavist has been designed to break this cycle and reduce craving and relapse. Nezavist acts on the vagus nerve at the gut level to create neural signals that counter the inflammation caused in the gut and the brain by the chronic consumption of alcohol. Nezavist changes the pattern of vagal-nerve signaling to the brain and vagal signals have been shown to suppress the microglial inflammatory state, thus reducing the craving for alcohol, supporting the maintenance of healthy brain tissue and aiding abstinence.

